Cookies help us deliver our services. By using our services, you agree to our use of cookies.
You have no items in your shopping cart.
Test Kits
Sort by
Display per page
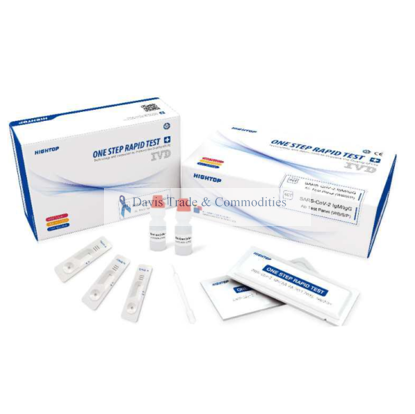
One Step SARS-CoV-2 (COVID-19) Rapid Test Kit
TK1001TM10
CE marked and FDA 510(K) Number K192123. The SARS-CoV-2 IgM/IgG Ab Rapid Test is used to detect the IgM and IgG antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human serum, plasma, or whole blood sample qualitatively. It is to be used as an aid in the diagnosis of infectious disease. 40T/box, 48 boxes/ctn
$8.23 excluding shipping
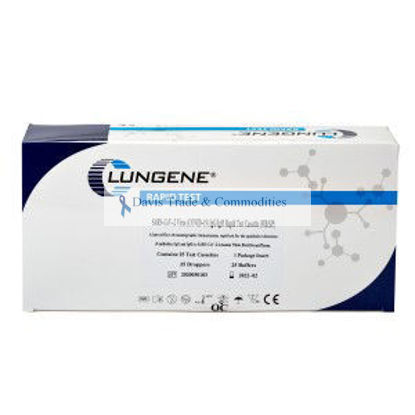
Clungene® SARS CoV-2 Virus Test Kit
TK1034AB14
Clungene® Coronavirus SARS CoV-2 Virus (COVID-19) IgG/IgM Rapid Test Kit FDA EUA Submission Number: EUA201121 There are 25 test kits in each box
$25.00 excluding shipping